Our math curriculum (Math U See) uses manipulatives on occasion, and we read honest-to-goodness books—no e-readers—for our grammar curriculum, but nothing is quite so tactile as our science work. The science guide that we use lays out suggested lessons that lean heavily on the Socratic method with at least one hands-on demonstration that follows the "show don't tell" principle.
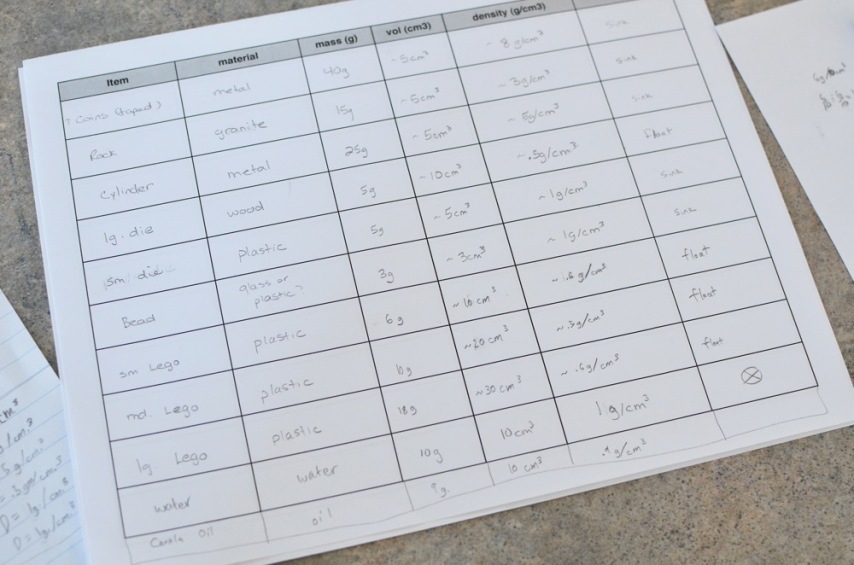
We were learning this week about density. We started with the definition of density, as grams per cm3, then discovered the meaning of that definition through a great "does it sink or float?" experiment. We collected a variety of objects all about the same volume. We measured their weight in grams on our kitchen scale, then, via Socratic method, decided on a means of measuring their volumes: through water displacement. Then, using good old traditional math we calculated each object's density. We also noted whether each item sank, or floated, in which case we used a straw to push it down into the water.
Finally, again via Socratic method, we discussed the pattern and discovered the difference between sinking and floating objects. What might the ultimate difference be? Calvin's hypothesis was that items with a denisity great than that of water sank. We decided on measuring the density of water, and discovered, of course, that his hypothesis was true, AND that the density of water was exactly 1g/1cm3.
It's all in the definition.